Clinical Trials Outsourcing Market Size and Forecast (2025 - 2033), Global and Regional Growth, Trend, Share and Industry Analysis Report Coverage: By Outsourcing Type (Functional Service Providers (FSP), Full-Service Outsourcing and Hybrid Models); By Service Type (Clinical Trial Data Management Services, Laboratory Services, Patient Recruitment, Site Management, Regulatory Affairs and Others); By Phase (Phase I, Phase II, Phase III and Phase IV); By Therapeutic Area (Oncology, Cardiovascular Disease, Infectious Disease, Neurology and Others); By End-user (Pharmaceutical Companies, Biopharmaceutical Companies, Academic and Research Institutions) and Geography
2025-08-05
Healthcare
Description
Clinical
Trials Outsourcing Market Overview
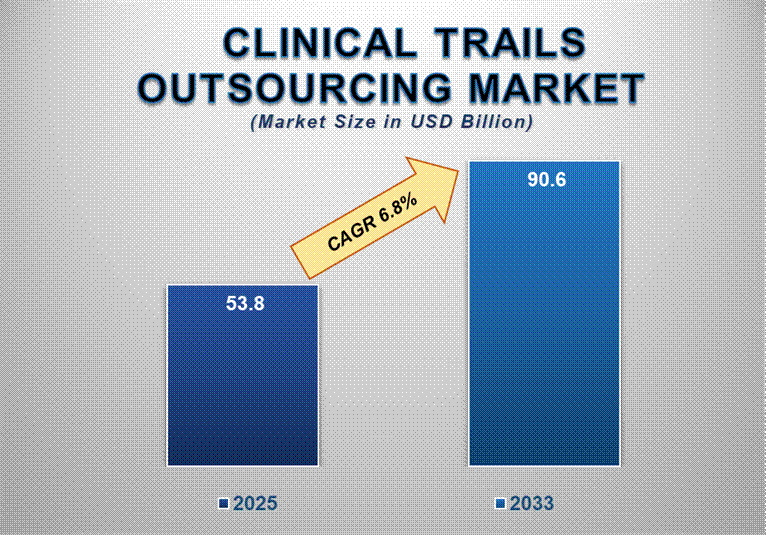
The Clinical Trials Outsourcing Market is set to witness remarkable growth from 2025 to 2033, driven by an increase in the number of oncology clinical trials globally, boosting the clinical trials outsourcing market. The Clinical trials outsourcing market at around USD 53.8 billion in 2025, and the market is expected to reach around USD 90.6 billion by 2033 at a healthy compound annual growth rate of 6.8% over a decade.
Clinical trial outsourcing is a
strategic practice where pharmaceutical, medical device, and biotechnology
firms outsource some aspects of clinical research to external service partners,
often termed as Contract Research Organizations (CROs). The model has become
increasingly popular over the last twenty years because of rising drug
development costs, complex regulatory demands, and demands for global trial
conduct. Outsourcing clinical trial processes like patient recruitment, data
management, regulatory compliance, and monitoring at clinical trial sites
enables the sponsor to access specialized skills, advanced tools, and
established networks in varying geographic regions. This not only streamlines
operational flow but also delivers time-to-market for new treatments faster.
Outsourcing enables companies to
concentrate on research and innovation as core competencies while maintaining
compliance with new international regulations. Furthermore, CROs tend to have
experience in dealing with regulatory systems across more than one country,
which is a critical component in the execution of multinational trials. The
market for clinical trials is driven by the increased number of clinical
trials, rising R&D investments, and the growth of personalized medicines.
It also imposes risks such as concerns with data security, quality control, as
well as reliance on external parties. In light of these risks, the use of
hybrid models mixing in-house capacity with external services is on the rise as
sponsors look to offset these risks. Overall, clinical trials outsourcing is an
important component of contemporary drug development in that it offers
cost-saving solutions that also increase flexibility and enable global trial
conduct, ultimately enabling faster delivery of effective and safe treatments
to global patients.
Clinical
Trials Outsourcing Market Drivers and Opportunities
Rising
R&D Expenditure is anticipated to lift the Clinical Trials Outsourcing
Market during the forecast period
A major driver of the clinical
trials outsourcing market is the steady rise in research and development
(R&D) spending of the pharmaceutical and biotechnology firms. As the cost
of bringing a new drug to market continues to increase—quite often to well over
a billion dollars—firms are looking to reduce the cost of the clinical trial
process while still maintaining its efficiency. Outsourcing to Contract
Research Organizations (CROs) enables sponsors to maximize their budgets by
using the specialized infrastructure, talent, and technological platforms of
the external service suppliers.
Also, outsourcing saves fixed
expenditures on in-house research staff and facilities. This cost flexibility
is particularly important to smaller and mid-sized biotechnology companies that
cannot afford to conduct major trials in-house. Given the worldwide drive
toward innovation and new medicines, particularly in oncology, neurology, and
the treatment of orphan diseases, rising research and development expenditure
is likely to further drive demand for outsourced clinical trials.
Increasing
Complexity of Clinical Trials drives the global Clinical Trials Outsourcing
Market
The increase in complexity of
clinical trials is the prime growth driver of the outsourcing market. Today's
trials tend to have large sample sizes, various study arms, adaptive trial
designs, and the employment of biomarkers or genomic data to support
personalized medicine. These complexities require sophisticated data analytics,
specialized recruitment strategies for patients, and advanced regulatory
navigation, which can be better managed by veteran CROs. Furthermore, the
increase in decentralized and virtual trials—particularly in the post-COVID-19
era—calls for robust digital infrastructure and the ability to conduct
monitoring from a distance. CROs usually possess the tools and the know-how to
execute these complex protocols in an effective and scalable way. SolarWinds
intelligence encourages sponsors to outsource clinical operations so that they
can meet tight timelines without jeopardizing compliance and the standard of
work. Increasingly technically complex and geographically fragmented trials
make outsourcing an operational solution to sidestep operational issues and
speed up the development pipeline.
Opportunity
for the Clinical Trials Outsourcing Market
Expansion
into emerging markets is a significant opportunity in the global clinical
trials outsourcing market
One of the key opportunities in
the market for clinical trials offshoring is the growth in emerging markets
like Brazil, China, India, and Eastern European markets. They boast a vast and
heterogeneous population of patients, reduced operating expenses, and enhanced
health infrastructure—making them sound locations to conduct clinical trials.
In addition to this, the governments of numerous emerging markets are also
investing heavily in their regulatory structure and trial landscape in a bid to
entice global studies.
This provides sponsors and CROs
with an opportunity to speed up patient recruitment, increase trial diversity,
and lower the cost of trials, while reaching a wider global audience. It can
also provide more speedy regulatory approvals and treatment-naïve access to
populations, which can become critical in some indications. Expanding
successfully, however, involves overcoming local regulations and ethics, which
established CROs are well-positioned to handle. As the need for global clinical
trial capacity evolves, emerging markets offer a strategic option for industry
players to become more competitive and speed drug development cycles.
Clinical
Trials Outsourcing Market Scope
|
Report Attributes |
Description |
|
Market Size in 2025 |
USD 53.8 Billion |
|
Market Forecast in 2033 |
USD 90.6 Billion |
|
CAGR% 2025-2033 |
6.8% |
|
Base Year |
2024 |
|
Historic Data |
2020-2024 |
|
Forecast Period |
2025-2033 |
|
Report USP |
Production, Consumption, company share, company heatmap, company
production Capacity, growth factors and more |
|
Segments Covered |
●
By Outsourcing Type ●
By Service Type ●
By Phase ●
By Therapeutic Type ●
By End-user |
|
Regional Scope |
●
North America ●
Europe ●
APAC ●
Latin America ●
Middle East and Africa |
|
Country Scope |
1)
U.S. 2)
Canada 3)
U.K. 4)
Germany 5)
France 6)
Italy 7)
Spain 8)
Netherland 9)
China 10)
India 11)
Japan 12)
South Korea 13)
Australia 14)
Mexico 15)
Brazil 16)
Argentina 17)
Saudi Arabia 18)
UAE 19) South Africa |
Clinical
Trials Outsourcing Market Report Segmentation Analysis
The global Clinical Trials Outsourcing Market industry analysis is segmented by Outsourcing Type, by Service Type, by phase, by Therapeutic Type, by End-user, and by region.
The
functional service providers (FSP) outsourcing types segment holds the largest
share in the clinical trials outsourcing market
The Functional Service Provider (FSP) model has emerged as the go-to model in clinical trials owing to its flexibility, cost-effectiveness, and scalability. According to the model, sponsors outsource discrete functions like data management, biostatistics, pharmacovigilance, or clinical monitoring to specialized service providers. The model enables pharmaceutical and biotechnology companies to maintain strategic control while minimizing operational burdens and fixed expenditures. The FSP model encourages long-term collaboration and integration with in-house teams, which results in better resource use and performance standards. Its adaptation to varying work volumes and access to global talent pools makes the model quite appealing, especially in large-scale and multi-regional trials. As clinical development gets more complex and resource-hungry, the FSP model of outsourcing picks up pace in the industry, owning the largest market share of the different types of outsourcing in clinical trials. This is likely to become more robust as the sponsors look for increased efficiency and flexibility in their R&D processes.
Clinical
Trial Data Management Service Type segment holds a major share in the Clinical
Trials Outsourcing Market
Clinical trial data management is
an essential part of clinical research with the aim of ensuring collected data
is accurate, complete, and in line with the requirements of the regulators. Due
to the growth in electronic data capture (EDC), decentralized trials, and
increased regulatory oversight, the demand for specialized data management
services has grown exponentially. Outsourcing this activity enables sponsors to
avail themselves of advanced technology, qualified people, as well as
established processes from specialized vendors and contract research
organizations (CROs).
Services provided in this regard
comprise data entry, cleaning, validation, and the preparation of
submission-ready datasets. Smooth data management shortens the trial timeline,
enhances decision-making, as well as ensures data integrity—drivers of successful
regulatory submissions. Due to the high quantity as well as complexity of data
collected in current trials, especially in therapeutic categories such as
oncology and orphan diseases, this segment contributes significantly to the
market for clinical trial outsourcing. With the growth in digital health
technologies, clinical trial data management will continue to be a central core
service in the market, further entrenching its market leadership.
Phase 3
segment holds a major share in the Clinical Trials Outsourcing Market
Phase III clinical trials are the largest, longest-lasting, and most resource-intensive phase of drug development, with large populations of patients in many different sites and often in many different countries involved. These trials are conducted to confirm efficacy, track adverse effects, and contrast the new medication with current standard treatments. Because of their scope and complexity, however, Phase III trials are typically farmed out to Contract Research Organizations (CROs) with global infrastructure, regulatory acumen, and operational capacity needed to complete them. The exorbitant cost of conducting Phase III trials—often more than half of the total cost of clinical development—motivates sponsors to look to outsourcing partners with the ability to maximize timelines and lower costs. Patient recruitment, site management, regulatory compliance, and data gathering are critical tasks performed in this phase by the CRO. For this reason, the Phase III segment of the market for clinical trials outsourcing is the largest and is maintaining its current dominance because of the critical nature and investment requirements of late-stage clinical development.
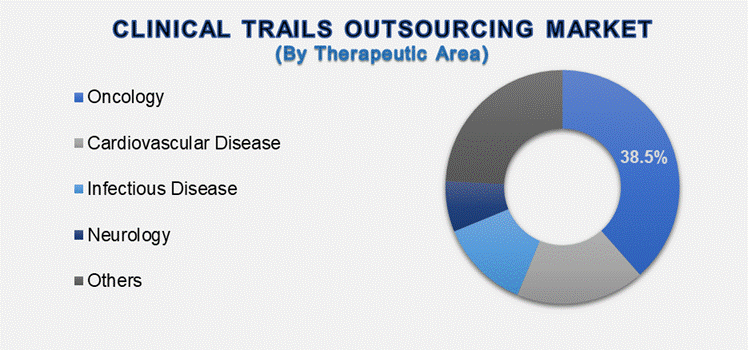
The Oncology
Therapeutic Area segment holds a major share in the Clinical Trials Outsourcing
Market
The oncology therapeutic area is
the market leader in clinical trials outsourcing because of the widespread
prevalence of cancer globally, the continuing need for new treatments, and
significant investments by pharmaceutical and biotechnology firms in research
and development. Oncology trials are intricate, with long study periods,
specialized investigators, biomarker development, and adaptive trial designs.
These characteristics make sponsors increasingly dependent on partners with
in-depth oncology knowledge and infrastructure.
In addition, the emergence of personalized medicine and immuno-oncology has contributed to more biomarker-driven investigations and companion diagnostic trials, further increasing the demand for specialized support. Oncology clinical trials also experience difficulties in regulatory compliance as well as patient recruitment across multiple geographies. Oncology-experienced Contract Research Organizations (CROs) mitigate these issues through strategic input as well as operational support. Due to the number and complexity of the oncology trials, the therapeutic area still controls the biggest clinical trials market share, a trend projected to endure as the research to treat cancer continues to evolve.
The following segments are part
of an in-depth analysis of the global Clinical Trials Outsourcing Market:
|
Market Segments |
|
|
By Outsourcing Type
|
●
Functional Service
Providers (FSP) ●
Full-Service
Outsourcing ●
Hybrid Models |
|
By Service Type |
●
Clinical Trial Data
Management Services ●
Laboratory Services ●
Patient Recruitment ●
Site Management ●
Regulatory Affairs ●
Others |
|
By Phase |
●
Phase I ●
Phase II ●
Phase III ●
Phase IV |
|
By Therapeutic Type |
●
Oncology ●
Cardiovascular
Disease ●
Infectious Disease ●
Neurology ●
Others |
|
By End-user |
●
Pharmaceutical
Companies ●
Biopharmaceutical
Companies ●
Academic and
Research Institutions |
Clinical
Trials Outsourcing Market Share Analysis by Region
The Asia
Pacific region is significantly growing at the fastest CAGR in the global
Clinical Trials Outsourcing Market over the forecast period.
The fastest compound annual
growth rate (CAGR) in the market for global clinical trials is being witnessed
in the Asia Pacific region, which is being fueled by the convergence of
positive factors, making the area a more desirable place for research to be
conducted. The growth is mainly being contributed to by the size, diversity,
and treatment-naïve population of the region, which ensures faster recruitment
of patients as well as shortened overall trial times. China, India, South
Korea, and Australia are the forerunners there with cost-effective conduct of
the trials, well-qualified medical professionals, and better regulatory
procedures. China and India reforms specifically have streamlined the process
of obtaining approval for clinical trials, greatly enticing multinational
sponsors as well as worldwide Contract Research Organizations (CROs) to
increase their footprint in the area.
Also, the Asia Pacific has the
advantage of robust government backing in the form of investment in clinical
research capabilities and healthcare infrastructure. Increased awareness of
lifestyle and chronic diseases, as well as their rising prevalence, is also
creating a larger number of suitable and willing participants. Furthermore, the
acceptance of digital health technologies and the proliferation of
decentralized clinical trials also serve to augment operational efficiencies.
Pharmaceutical and biotechnology firms wanting to reduce costs while tapping
new markets of patients, the Asia Pacific is set to be the main driver in the
destiny of global clinical trials offshoring.
Clinical
Trials Outsourcing Market Competition Landscape Analysis
The market is
competitive, with several established players and new entrants offering a range
of clinical trials outsourcing services. Some of the key players include IQVIA,
Labcorp Drug Development, ICON plc, Syneos Health, Parexel International,
Charles River Laboratories, Medpace, and others
Global
Clinical Trials Outsourcing Market Recent Developments News:
- In February 2025, Veeda Clinical Research of India
acquired Heads, a European oncology clinical trials-focused CRO. The
strategic acquisition gives Veeda a wider global reach with access to 25
operating locations in Europe, North America, and the Asian Pacific.
- In April of 2024, Parexel, a global leader in the
Contract Research Organization (CRO) industry, announced a multi-year
partnership with Palantir Technologies. The agreement is to incorporate
the power of Palantir's advanced AI and data analytics to enhance data
management solutions in clinical trials.
- In July 2024, the global CRO with expertise in
biostatistics and data management, Phastar, joined forces with Beaconcure
to enhance collaboration in clinical trials. The collaboration is geared
towards enabling real-time sharing of data and minimizing iterative review
cycles, thus streamlining the process of clinical trials. Using Beaconcure
technology, Phastar hopes to increase the accuracy and efficiency of data
in clinical trials.
The
Global Clinical Trials Outsourcing Market is dominated by a few large
companies, such as
●
IQVIA
●
Labcorp Drug
Development
●
ICON plc
●
Syneos Health
●
Parexel International
●
Charles River
Laboratories
●
Medpace
●
PPD (Thermo Fisher
Scientific)
●
PRA Health Sciences
●
Wuxi AppTec
●
Covance Inc.
●
SGS SA
●
Pharmaceutical Product
Development, LLC
●
PSI CRO
●
KCR S.A.
● Other Prominent Players
Frequently Asked Questions
-
- Global Clinical Trials Outsourcing Market Introduction and Market Overview
- Objectives of the Study
- Global Clinical Trials Outsourcing Market Scope and Market Estimation
- Global Clinical Trials Outsourcing Market Overall Market Size (US$ Bn), Market CAGR (%), Market forecast (2025 - 2033)
- Global Clinical Trials Outsourcing Market Revenue Share (%) and Growth Rate (Y-o-Y) from 2021 - 2033
- Market Segmentation
- Outsourcing Type of Global Clinical Trials Outsourcing Market
- Service Type of Global Clinical Trials Outsourcing Market
- Phase of Global Clinical Trials Outsourcing Market
- Therapeutic Area of Global Clinical Trials Outsourcing Market
- End-user of Global Clinical Trials Outsourcing Market
- Region of Global Clinical Trials Outsourcing Market
- Executive Summary
- Demand Side Trends
- Key Market Trends
- Market Demand (US$ Bn) Analysis 2021 – 2024 and Forecast, 2025 – 2033
- Demand and Opportunity Assessment
- Demand Supply Scenario
- Market Dynamics
- Drivers
- Limitations
- Opportunities
- Impact Analysis of Drivers and Restraints
- Emerging Trends for Clinical Trials Outsourcing Market
- Porter’s Five Forces Analysis
- PEST Analysis
- Political Factors
- Economic Factors
- Social Factors
- Technology Factors
- Key Regulation
- Global Clinical Trials Outsourcing Market Estimates & Historical Trend Analysis (2021 - 2024)
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by Outsourcing Type
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Outsourcing Type, 2021 - 2033
- Functional Service Providers (FSP)
- Full-Service Outsourcing
- Hybrid Models
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Outsourcing Type, 2021 - 2033
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by Service Type
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Service Type, 2021 - 2033
- Clinical Trial Data Management Services
- Laboratory Services
- Patient Recruitment
- Site Management
- Regulatory Affairs
- Others
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Service Type, 2021 - 2033
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by Phase
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Phase, 2021 - 2033
- Phase I
- Phase II
- Phase III
- Phase IV
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Phase, 2021 - 2033
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by Therapeutic Area
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Therapeutic Area, 2021 - 2033
- Oncology
- Cardiovascular Disease
- Infectious Disease
- Neurology
- Others
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by Therapeutic Area, 2021 - 2033
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by End-user
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by End-user, 2021 - 2033
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Academic and Research Institutions
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by End-user, 2021 - 2033
- Global Clinical Trials Outsourcing Market Estimates & Forecast Trend Analysis, by region
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by region, 2021 - 2033
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
- Global Clinical Trials Outsourcing Market Revenue (US$ Bn) Estimates and Forecasts, by region, 2021 - 2033
- North America Clinical Trials Outsourcing Market: Estimates & Forecast Trend Analysis
- North America Clinical Trials Outsourcing Market Assessments & Key Findings
- North America Clinical Trials Outsourcing Market Introduction
- North America Clinical Trials Outsourcing Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Outsourcing Type
- By Service Type
- By Phase
- By Therapeutic Area
- By End-user
- By Country
- The U.S.
- Canada
- North America Clinical Trials Outsourcing Market Assessments & Key Findings
- Europe Clinical Trials Outsourcing Market: Estimates & Forecast Trend Analysis
- Europe Clinical Trials Outsourcing Market Assessments & Key Findings
- Europe Clinical Trials Outsourcing Market Introduction
- Europe Clinical Trials Outsourcing Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Outsourcing Type
- By Service Type
- By Phase
- By Therapeutic Area
- By End-user
- By Country
- Germany
- Italy
- K.
- France
- Spain
- Netherland
- Rest of Europe
- Europe Clinical Trials Outsourcing Market Assessments & Key Findings
- Asia Pacific Clinical Trials Outsourcing Market: Estimates & Forecast Trend Analysis
- Asia Pacific Market Assessments & Key Findings
- Asia Pacific Clinical Trials Outsourcing Market Introduction
- Asia Pacific Clinical Trials Outsourcing Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Outsourcing Type
- By Service Type
- By Phase
- By Therapeutic Area
- By End-user
- By Country
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- Asia Pacific Market Assessments & Key Findings
- Middle East & Africa Clinical Trials Outsourcing Market: Estimates & Forecast Trend Analysis
- Middle East & Africa Market Assessments & Key Findings
- Middle East & Africa Clinical Trials Outsourcing Market Introduction
- Middle East & Africa Clinical Trials Outsourcing Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Outsourcing Type
- By Service Type
- By Phase
- By Therapeutic Area
- By End-user
- By Country
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- Middle East & Africa Market Assessments & Key Findings
- Latin America Clinical Trials Outsourcing Market: Estimates & Forecast Trend Analysis
- Latin America Market Assessments & Key Findings
- Latin America Clinical Trials Outsourcing Market Introduction
- Latin America Clinical Trials Outsourcing Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Outsourcing Type
- By Service Type
- By Phase
- By Therapeutic Area
- By End-user
- By Country
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Latin America Market Assessments & Key Findings
- Country Wise Market: Introduction
- Competition Landscape
- Global Clinical Trials Outsourcing Market Product Mapping
- Global Clinical Trials Outsourcing Market Concentration Analysis, by Leading Players / Innovators / Emerging Players / New Entrants
- Global Clinical Trials Outsourcing Market Tier Structure Analysis
- Global Clinical Trials Outsourcing Market Concentration & Company Market Shares (%) Analysis, 2024
- Company Profiles
- IQVIA
- Company Overview & Key Stats
- Financial Performance & KPIs
- Product Portfolio
- SWOT Analysis
- Business Strategy & Recent Developments
- IQVIA
* Similar details would be provided for all the players mentioned below
- Labcorp Drug Development
- ICON plc
- Syneos Health
- Parexel International
- Charles River Laboratories
- Medpace
- PPD (Thermo Fisher Scientific)
- PRA Health Sciences
- Wuxi AppTec
- Covance Inc.
- SGS SA
- Pharmaceutical Product Development, LL
- PSI CRO
- KCR S.A.
- Other Prominent Players
- Research Methodology
- External Transportations / Databases
- Internal Proprietary Database
- Primary Research
- Secondary Research
- Assumptions
- Limitations
- Report FAQs
- Research Findings & Conclusion
- Global Clinical Trials Outsourcing Market Introduction and Market Overview
Our Research Methodology
"Insight without rigor is just noise."
We follow a comprehensive, multi-phase research framework designed to deliver accurate, strategic, and decision-ready intelligence. Our process integrates primary and secondary research , both quantitative and qualitative , along with dual modeling techniques ( top-down and bottom-up) and a final layer of validation through our proprietary in-house repository.
PRIMARY RESEARCH
Primary research captures real-time, firsthand insights from the market to understand behaviors, motivations, and emerging trends.
1. Quantitative Primary Research
Objective: Generate statistically significant data directly from market participants.
Approaches:- Structured surveys with customers, distributors, and field agents
- Mobile-based data collection for point-of-sale audits and usage behavior
- Phone-based interviews (CATI) for market sizing and product feedback
- Online polling around industry events and digital campaigns
- Purchase frequency by customer type
- Channel performance across geographies
- Feature demand by application or demographic
2. Qualitative Primary Research
Objective: Explore decision-making drivers, pain points, and market readiness.
Approaches:- In-depth interviews (IDIs) with executives, product managers, and key decision-makers
- Focus groups among end users and early adopters
- Site visits and observational research for consumer products
- Informal field-level discussions for regional and cultural nuances
SECONDARY RESEARCH
This phase helps establish a macro-to-micro understanding of market trends, size, regulation, and competitive dynamics, sourced from credible and public domain information.
1. Quantitative Secondary Research
Objective: Model market value and segment-level forecasts based on published data.
Sources include:- Financial reports and investor summaries
- Government trade data, customs records, and regulatory statistics
- Industry association publications and economic databases
- Channel performance and pricing data from marketplace listings
- Revenue splits, pricing trends, and CAGR estimates
- Supply-side capacity and volume tracking
- Investment analysis and funding benchmarks
2. Qualitative Secondary Research
Objective: Capture strategic direction, innovation signals, and behavioral trends.
Sources include:- Company announcements, roadmaps, and product pipelines
- Publicly available whitepapers, conference abstracts, and academic research
- Regulatory body publications and policy briefs
- Social and media sentiment scanning for early-stage shifts
- Strategic shifts in market positioning
- Unmet needs and white spaces
- Regulatory triggers and compliance impact
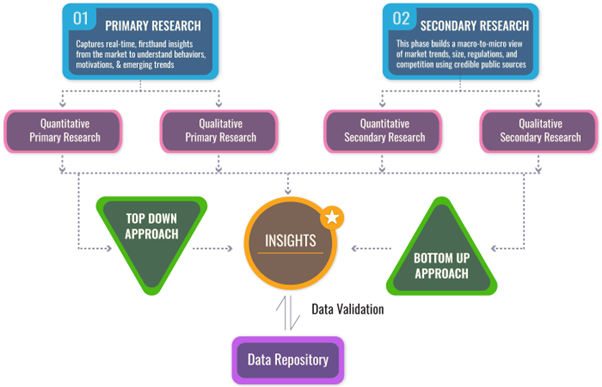
DUAL MODELING: TOP-DOWN + BOTTOM-UP
To ensure robust market estimation, we apply two complementary sizing approaches:
Top-Down Modeling:- Start with broader industry value (e.g., global or regional TAM)
- Apply filters by segment, geography, end-user, or use case
- Adjust with primary insights and validation benchmarks
- Ideal for investor-grade market scans and opportunity mapping
- Aggregate from the ground up using sales volumes, pricing, and unit economics
- Use internal modeling templates aligned with stakeholder data
- Incorporate distributor-level or region-specific inputs
- Most accurate for emerging segments and granular sub-markets
DATA VALIDATION: IN-HOUSE REPOSITORY
We close the loop with proprietary data intelligence built from ongoing projects, industry monitoring, and historical benchmarking. This repository includes:
- Multi-sector market and pricing models
- Key trendlines from past interviews and forecasts
- Benchmarked adoption rates, churn patterns, and ROI indicators
- Industry-specific deviation flags and cross-check logic
- Catches inconsistencies early
- Aligns projections across studies
- Enables consistent, high-trust deliverables

