In Vitro Diagnostics Market Size And Forecast (2020 - 2033), Global And Regional Growth, Trend, Share And Industry Analysis Report Coverage: By Product (Instruments, Reagents, Others), Technology (Immunoassay, Hematology, Clinical Chemistry, Molecular Diagnostics, Coagulation, Microbiology, Others), Application (Infectious Diseases, Diabetes, Oncology, Cardiology, Nephrology, Autoimmune Diseases, Drug Testing, Other Applications), End-User (Hospitals, Laboratories, Academic & Research Institutes, Point-Of-Care Testing Centers, Home Care Settings, Others) And Geography
2025-08-20
Healthcare
Description
In Vitro Diagnostics
Market Overview
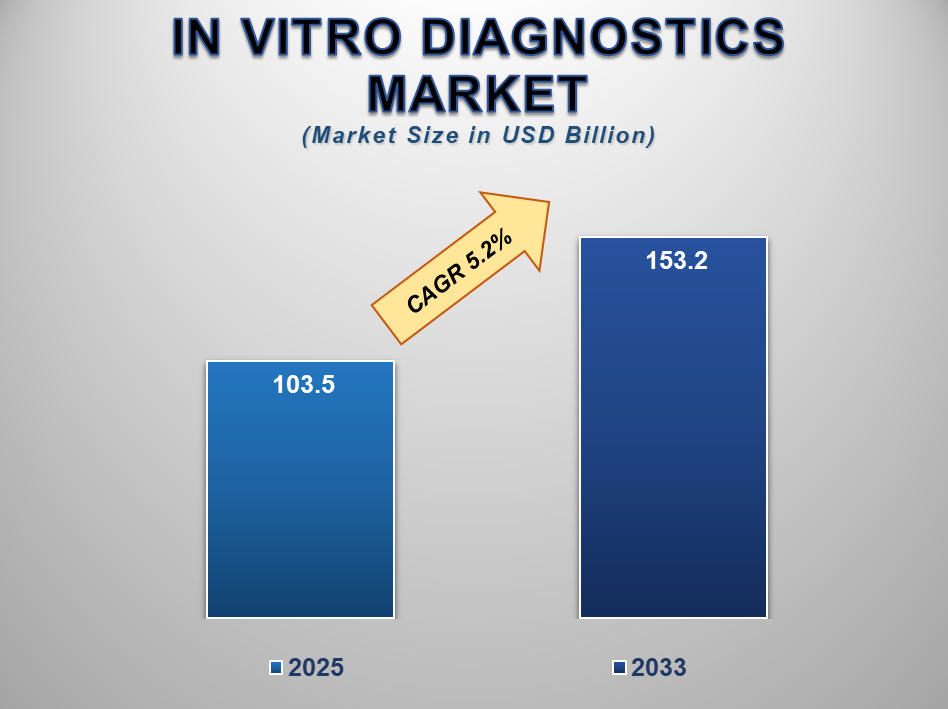
The global in vitro diagnostics market is poised for strong growth from 2025 to 2033. This increase is driven by advances in testing technology, the rise in chronic illnesses, and greater demand for early diagnosis. By 2025, the market is expected to reach about USD 103.5 billion. By 2033, it is projected to grow to around USD 153.2 billion. This growth marks an annual rate of 5.2% over the decade.
In vitro diagnostics involves
testing biological samples like blood, urine, and tissues. These tests help
diagnose diseases, monitor treatments, and check overall health. The market
covers many techniques, such as immunoassays, molecular tests, blood analysis,
and chemistry tests. Key factors shaping this market include technological
progress, the shift toward preventive care, point-of-care testing, and others.
The aging population, more prone to long-term illnesses, also boosts demand for
these tests. Automation and artificial intelligence are playing larger roles in
making diagnostics more accurate and efficient. The COVID-19 pandemic sped up
market growth due to high demand for PCR, antigen, and antibody tests. As
health systems adopt remote and digital testing methods, the market is expected
to keep growing. Emerging trends will influence future growth, including the
rise of self-testing kits and the expansion of molecular diagnostics. Changes
in regulations that make test approval easier will also impact the market.
North America leads the way now, thanks to strong healthcare systems and
widespread use of advanced testing tools. Meanwhile, the Asia-Pacific region is
set to see the fastest growth due to more healthcare spending and increased
awareness. As innovations continue and health needs change, the in vitro
diagnostics market will stay a vital part of the global healthcare industry.
In Vitro Diagnostics Market Drivers and Opportunities
The rising prevalence of chronic and infectious diseases is
expected to increase the in vitro diagnostics market demand during the forecast
period
The demand for in vitro
diagnostics is expected to rise as more chronic and infectious diseases emerge.
An increase in illnesses like cancer, heart disease, and diabetes is a primary
reason for this growth. According to the World Health Organization, nearly 71%
of deaths worldwide are caused by chronic conditions, highlighting the need for
early detection and ongoing checkups. The rise in diabetes, driven by lifestyle
changes, has boosted the need for glucose and HbA1c tests. Cancer cases are
also climbing, pushing demand for advanced tests like molecular diagnostics and
genetic screening to catch it early and plan treatments. Infectious diseases
like flu, tuberculosis, HIV/AIDS, and hepatitis keep fueling the need for quick
and reliable diagnostic tools. The COVID-19 pandemic sped up the use of tests
such as RT-PCR and antigen tests, attracting large investments to this market.
Governments and health groups everywhere focus on tracking and preventing
diseases, which adds to the need for better diagnostic tests. Increased
awareness about health and prevention also plays a role, as more people seek
regular checkups to find health issues early. All these factors work together
to shape the future of the in vitro diagnostics market, making it an essential
part of healthcare today.
Increasing demand for point-of-care and home testing is
driving the global in vitro diagnostics market growth
The need for point-of-care (POC)
and home-based testing is transforming the In Vitro Diagnostics (IVD) market.
Testing once depended on visiting labs and waiting days for results. Today, new
POC tools make real-time testing easier and more available. These quick tests
give results fast, so patients can get treatment right away. The rise in demand
for local healthcare options, especially in remote or underserved areas, has
led to more portable testing devices being used widely. The COVID-19 pandemic
sped up this shift, as millions relied on home antigen and PCR tests to detect
the virus. Self-monitoring tools for chronic conditions, like blood glucose
meters for diabetes and home pregnancy tests, have also grown popular. The
market will likely keep expanding through constant innovation in POC devices.
Companies are making smaller, simpler, and cheaper tests to meet this demand.
At the same time, more people are aware of health issues and want to take
preventive steps. Governments and clinics support this trend by creating
policies that favor home testing. This helps ease pressure on hospitals and
clinics. As smartphone-based testing and AI analysis improve, the direction of
the global In Vitro Diagnostics Market IVD
market favors more patient-focused care. Healthcare is becoming more
convenient, affordable, and efficient as new tech advances continue.
Integration of digital health and AI in diagnostics is poised
to create significant opportunities in the global in vitro diagnostics market
The use of digital health tools
and artificial intelligence (AI) in diagnostics is shaping the trends in the In
Vitro Diagnostics market. AI improves testing accuracy by analyzing large
amounts of data and spotting disease patterns with great precision. Machine
learning is being used in imaging, pathology, and molecular tests to catch
diseases early. These AI tools also help doctors make faster, better decisions
and lower the chance of mistakes. Remote diagnostics are becoming easier thanks
to telemedicine and cloud-based data sharing, especially in rural areas.
Automated workflows in labs powered by AI make testing faster and more
efficient. Companies that focus on AI-driven diagnostics are likely to stay
ahead as digital health continues to change the industry. As more people adopt
AI and digital tools, the market for in-vitro diagnostics will grow largely due
to new, smarter testing solutions.
In Vitro Diagnostics Market Scope
|
Report Attributes |
Description |
|
Market Size in 2025 |
USD 103.5 Billion |
|
Market Forecast in 2033 |
USD 153.2 Billion |
|
CAGR % 2025-2033 |
5.2% |
|
Base Year |
2024 |
|
Historic Data |
2020-2024 |
|
Forecast Period |
2025-2033 |
|
Report USP |
Production, Consumption, company share, company heatmap, company
production capacity, growth factors and more |
|
Segments Covered |
●
By Product ●
By Technology ●
By Application ●
By End-use |
|
Regional Scope |
●
North America ●
Europe ●
APAC ●
Latin America ●
Middle East and Africa |
|
Country Scope |
1)
U.S. 2)
Canada 3)
Germany 4)
UK 5)
France 6)
Spain 7)
Italy 8)
Switzerland 9)
China 10)
Japan 11)
India 12)
Australia 13)
South Korea 14)
Brazil 15)
Mexico 16)
Argentina 17)
South Africa 18)
Saudi Arabia 19) UAE |
In Vitro Diagnostics Market Report Segmentation Analysis
The global in vitro diagnostics
market industry analysis is segmented by product, by technology, by
application, by end-use, and by region.
The reagents segment is anticipated to hold the highest share
of the global In Vitro Diagnostics market during the projected timeframe.
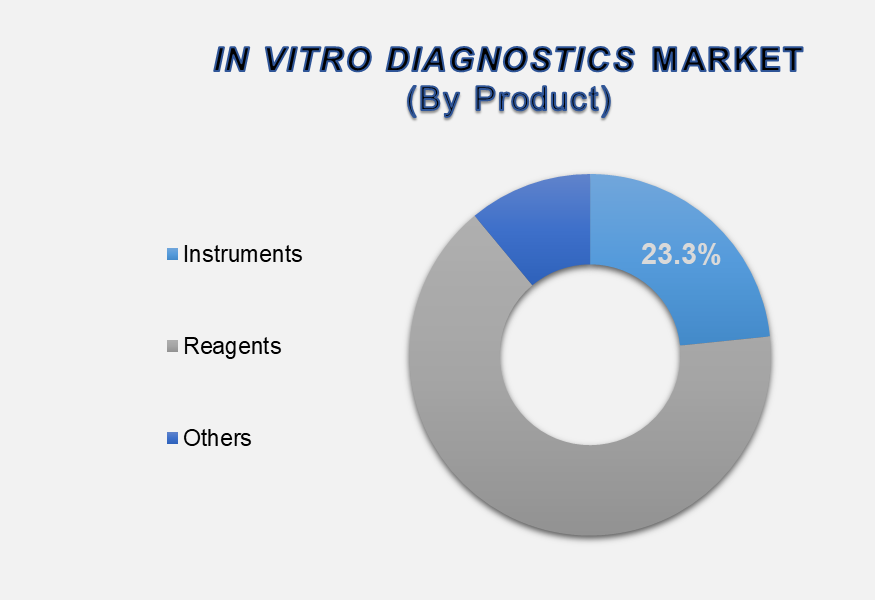
Based on product, the market is segmented into instruments, reagents, and others. The reagents segment dominates the market with a 65.4% share due to the increasing demand for diagnostic tests, advancements in molecular and immunoassay reagents, and the rising prevalence of chronic and infectious diseases. Reagents are essential components of diagnostic tests, used in various laboratory and point-of-care testing procedures, and their frequent use in high-throughput testing has contributed significantly to the growth of the In Vitro Diagnostics market.
The immunoassay dominated the market in 2025 and is predicted
to grow at the highest CAGR over the forecast period.
Based on technology, the market
is segmented into immunoassay, hematology, clinical chemistry, molecular
diagnostics, coagulation, microbiology, and others. Among these, the
immunoassay segment has the largest market share in the In Vitro Diagnostics
market due to its widespread application in disease diagnosis, biomarker
testing, and therapeutic drug monitoring. The In Vitro Diagnostics market is
growing in this segment on the back of increasing demand for rapid and accurate
diagnostic testing, increasing prevalence of chronic diseases such as cancer
and cardiovascular diseases, and growing adoption of point-of-care testing. In
vitro diagnostics market analysis highlights continued innovation in
immunoassay technologies, such as chemiluminescent immunoassays (CLIA) and
enzyme-linked immunosorbent assays (ELISA), which enhance sensitivity and
specificity, driving increased use in clinical and laboratory settings.
The infectious diseases segment is expected to drive the
global in vitro diagnostics market growth.
On the basis of application, the
market is segmented into infectious diseases, diabetes, oncology, cardiology,
nephrology, autoimmune diseases, drug testing, and other applications. The
infectious diseases segment has the biggest share in the In Vitro Diagnostics
market due to the rising incidence of bacterial, viral, and fungal infections
worldwide. The In Vitro Diagnostics market in this segment is significantly
driven by the high prevalence of infectious diseases such as COVID-19,
influenza, tuberculosis, hepatitis, and sexually transmitted diseases (STDs).
The demand for quick, precise, and low-cost diagnostic solutions has led to
immense technological advancements in molecular and immunodiagnostic
technologies, which have further supported the uptake of infectious disease
testing.
The Hospitals segment is expected to dominate the market
during the forecast period.
Based on end-use, the market is
segmented into hospitals, laboratories, academic & research institutes,
point-of-care testing centers, home care settings, and others. The hospitals
segment accounted for the largest share of the In Vitro Diagnostics market in
2025. High patient flow, increasing demand for early disease diagnosis, and
availability of advanced diagnostic technologies in hospitals are the factors
driving the growth. The In Vitro Diagnostics market in this segment is
propelled mainly by the growing incidences of chronic and infectious diseases,
increasing healthcare expenditure, and the need for quick and accurate
diagnostic results for effective treatment planning. Hospitals heavily rely on
in-house diagnostic laboratories with high-throughput and automated testing
systems for effective patient management
The following segments are part of an in-depth analysis of the global
In Vitro Diagnostics market:
|
Market Segments |
|
|
By Product |
●
Instruments ●
Reagents ●
Others |
|
By Technology |
●
Immunoassay ●
Hematology ●
Clinical Chemistry ●
Molecular
Diagnostics ●
Coagulation ●
Microbiology ●
Others |
|
By Application |
●
Infectious Diseases ●
Diabetes ●
Oncology ●
Cardiology ●
Nephrology ●
Autoimmune Diseases ●
Drug Testing ●
Other applications |
|
By End-use |
●
Hospitals ●
Laboratories ●
Academic &
Research Institutes ●
Point-of-Care
Testing Centers ●
Home Care Settings ●
Others |
In Vitro Diagnostics Market Share Analysis by Region
North America is projected to hold the largest share of the
global In Vitro Diagnostics market over the forecast period.
North America is the biggest
market for In Vitro Diagnostics and is expected to hold the largest market
share of 49.2%. Growth is driven by well-developed healthcare infrastructure,
high adoption of sophisticated diagnostic technologies, and a rising burden of
chronic and infectious diseases. In Vitro Diagnostics market growth in North
America is largely contributed to by the presence of leading market players,
continuous investment in research and development (R&D), and a strong
regulatory framework ensuring the quality and efficiency of diagnostic
solutions. The increasing rate of diseases such as cancer, diabetes,
cardiovascular conditions, and infectious diseases has accelerated the demand
for rapid and accurate diagnostic tests, further driving market growth in the
region.
However, the Asia-Pacific (APAC)
region is expected to witness the highest CAGR in the Asia-Pacific In Vitro
Diagnostics market during the forecast period. The region's rapidly growing
market is attributed to increasing healthcare investments, enhancing awareness
about early disease diagnosis, and the rising burden of chronic and infectious
diseases. China, India, and Japan are the nations that are experiencing high
growth in diagnostic testing due to their emerging healthcare infrastructure, enhanced
disposable income, and increasing demand for cost-effective and accessible
diagnostic solutions. The high patient pool, government-led healthcare
initiatives, and growth in molecular and point-of-care diagnostics are also
augmenting the In Vitro Diagnostics market trends in APAC, rendering it a
high-growth market in the IVD sector worldwide.
In Vitro Diagnostics Market Competition Landscape Analysis
The in vitro diagnostics market
is poised for significant growth, with key players investing heavily in
technology and infrastructure. These companies are actively engaged in research
and development, strategic partnerships, and large-scale project implementations
to enhance their market positions.
Global In Vitro Diagnostics Market Recent Developments News:
- In February 2025, ABL Diagnostics will manufacture
and sell a full portfolio of UltraGene PCR tests bought from its parent
company, Advanced Biological Laboratories. The tests are for over 100
pathogens, spanning infectious disease diagnostics across various
conditions. The firm will pair PCR with its DeepChek sequencing products,
expanding its presence in precision medicine.
- In January 2025, QIAGEN gained U.S. FDA clearance
for its QIAstat-Dx Gastrointestinal Panel 2 Mini B&V, a syndromic test
for bacterial and viral gastrointestinal infections. The company is
planning a product launch to expand QIAGEN's syndromic testing portfolio
to offer comprehensive and focused solutions for improving inpatient and
outpatient diagnostics.
- In October 2024, Health Canada approved Becton, Dickinson and Company
(BD) Onclarity HPV Assay for use with self-collected vaginal samples for
home testing of human papillomavirus (HPV).
The Global In Vitro
Diagnostics Market is dominated by a few
large companies, such as
●
Roche Diagnostics
●
Abbott Laboratories
●
Siemens Healthineers
●
Danaher Corporation
●
Thermo Fisher Scientific
●
Bio-Rad Laboratories
●
Qiagen N.V.
●
bioMérieux SA
●
Sysmex Corporation
●
Becton, Dickinson and Company (BD)
●
Hologic, Inc.
●
Ortho Clinical Diagnostics
●
Luminex Corporation
●
PerkinElmer, Inc.
● Others
Frequently Asked Questions
- Global In Vitro Diagnostics Market Introduction and Market Overview
- Objectives of the Study
- Global In Vitro Diagnostics Market Scope and Market Estimation
- Global In Vitro Diagnostics Overall Market Size (US$ Bn), Market CAGR (%), Market forecast (2025 - 2033)
- Global In Vitro Diagnostics Market Revenue Share (%) and Growth Rate (Y-o-Y) from 2021 - 2033
- Market Segmentation
- Product of Global In Vitro Diagnostics Market
- Technology of Global In Vitro Diagnostics Market
- Application of Global In Vitro Diagnostics Market
- End-use of Global In Vitro Diagnostics Market
- Region of Global In Vitro Diagnostics Market
- Executive Summary
- Demand Side Trends
- Key Market Trends
- Market Demand (US$ Bn) Analysis 2021 – 2024 and Forecast, 2025 – 2033
- Demand and Opportunity Assessment
- Demand Supply Scenario
- Market Dynamics
- Drivers
- Limitations
- Opportunities
- Impact Analysis of Drivers and Restraints
- Emerging Trends for In Vitro Diagnostics Market
- Porter’s Five Forces Analysis
- PEST Analysis
- Key Regulation
- Global In Vitro Diagnostics Market Estimates & Historical Trend Analysis (2021 - 2024)
- Global In Vitro Diagnostics Market Estimates & Forecast Trend Analysis, by Product
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Product, 2021 - 2033
- Instruments
- Reagents
- Others
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Product, 2021 - 2033
- Global In Vitro Diagnostics Market Estimates & Forecast Trend Analysis, by Technology
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Technology, 2021 - 2033
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostics
- Coagulation
- Microbiology
- Others
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Technology, 2021 - 2033
- Global In Vitro Diagnostics Market Estimates & Forecast Trend Analysis, by Application
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Application, 2021 - 2033
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Diseases
- Drug Testing
- Other applications
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Application, 2021 - 2033
- Global In Vitro Diagnostics Market Estimates & Forecast Trend Analysis, by End-use
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by End-use, 2021 - 2033
- Hospitals
- Laboratories
- Academic & Research Institutes
- Point-of-Care Testing Centers
- Home Care Settings
- Others
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by End-use, 2021 - 2033
- Global In Vitro Diagnostics Market Estimates & Forecast Trend Analysis, by Region
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Region, 2021 - 2033
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
- Global In Vitro Diagnostics Market Revenue (US$ Bn) Estimates and Forecasts, by Region, 2021 - 2033
- North America In Vitro Diagnostics Market: Estimates & Forecast Trend Analysis
- North America In Vitro Diagnostics Market Assessments & Key Findings
- North America In Vitro Diagnostics Market Introduction
- North America In Vitro Diagnostics Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Product
- By Technology
- By Application
- By End-use
- By Country
- The U.S.
- Canada
- North America In Vitro Diagnostics Market Assessments & Key Findings
- Europe In Vitro Diagnostics Market: Estimates & Forecast Trend Analysis
- Europe In Vitro Diagnostics Market Assessments & Key Findings
- Europe In Vitro Diagnostics Market Introduction
- Europe In Vitro Diagnostics Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Product
- By Technology
- By Application
- By End-use
- By Country
- Germany
- Italy
- K.
- France
- Spain
- Switzerland
- Rest of Europe
- Europe In Vitro Diagnostics Market Assessments & Key Findings
- Asia Pacific In Vitro Diagnostics Market: Estimates & Forecast Trend Analysis
- Asia Pacific Market Assessments & Key Findings
- Asia Pacific In Vitro Diagnostics Market Introduction
- Asia Pacific In Vitro Diagnostics Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Product
- By Technology
- By Application
- By End-use
- By Country
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- Asia Pacific Market Assessments & Key Findings
- Middle East & Africa In Vitro Diagnostics Market: Estimates & Forecast Trend Analysis
- Middle East & Africa Market Assessments & Key Findings
- Middle East & Africa In Vitro Diagnostics Market Introduction
- Middle East & Africa In Vitro Diagnostics Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Product
- By Technology
- By Application
- By End-use
- By Country
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- Middle East & Africa Market Assessments & Key Findings
- Latin America In Vitro Diagnostics Market: Estimates & Forecast Trend Analysis
- Latin America Market Assessments & Key Findings
- Latin America In Vitro Diagnostics Market Introduction
- Latin America In Vitro Diagnostics Market Size Estimates and Forecast (US$ Billion) (2021 - 2033)
- By Product
- By Technology
- By Application
- By End-use
- By Country
- Brazil
- Argentina
- Mexico
- Rest of LATAM
- Latin America Market Assessments & Key Findings
- Country Wise Market: Introduction
- Competition Landscape
- Global In Vitro Diagnostics Market Product Mapping
- Global In Vitro Diagnostics Market Concentration Analysis, by Leading Players / Innovators / Emerging Players / New Entrants
- Global In Vitro Diagnostics Market Tier Structure Analysis
- Global In Vitro Diagnostics Market Concentration & Company Market Shares (%) Analysis, 2023
- Company Profiles
- Roche Diagnostics
- Company Overview & Key Stats
- Financial Performance & KPIs
- Product Portfolio
- SWOT Analysis
- Business Strategy & Recent Developments
- Roche Diagnostics
* Similar details would be provided for all the players mentioned below
- Abbott Laboratories
- Siemens Healthineers
- Danaher Corporation
- Thermo Fisher Scientific
- Bio-Rad Laboratories
- Qiagen N.V.
- bioMérieux SA
- Sysmex Corporation
- Becton, Dickinson and Company (BD)
- Hologic, Inc.
- Ortho Clinical Diagnostics
- Luminex Corporation
- PerkinElmer, Inc.
- Others
- Research Methodology
- External Transportations / Databases
- Internal Proprietary Database
- Primary Research
- Secondary Research
- Assumptions
- Limitations
- Report FAQs
- Research Findings & Conclusion
Our Research Methodology
"Insight without rigor is just noise."
We follow a comprehensive, multi-phase research framework designed to deliver accurate, strategic, and decision-ready intelligence. Our process integrates primary and secondary research , both quantitative and qualitative , along with dual modeling techniques ( top-down and bottom-up) and a final layer of validation through our proprietary in-house repository.
PRIMARY RESEARCH
Primary research captures real-time, firsthand insights from the market to understand behaviors, motivations, and emerging trends.
1. Quantitative Primary Research
Objective: Generate statistically significant data directly from market participants.
Approaches:- Structured surveys with customers, distributors, and field agents
- Mobile-based data collection for point-of-sale audits and usage behavior
- Phone-based interviews (CATI) for market sizing and product feedback
- Online polling around industry events and digital campaigns
- Purchase frequency by customer type
- Channel performance across geographies
- Feature demand by application or demographic
2. Qualitative Primary Research
Objective: Explore decision-making drivers, pain points, and market readiness.
Approaches:- In-depth interviews (IDIs) with executives, product managers, and key decision-makers
- Focus groups among end users and early adopters
- Site visits and observational research for consumer products
- Informal field-level discussions for regional and cultural nuances
SECONDARY RESEARCH
This phase helps establish a macro-to-micro understanding of market trends, size, regulation, and competitive dynamics, sourced from credible and public domain information.
1. Quantitative Secondary Research
Objective: Model market value and segment-level forecasts based on published data.
Sources include:- Financial reports and investor summaries
- Government trade data, customs records, and regulatory statistics
- Industry association publications and economic databases
- Channel performance and pricing data from marketplace listings
- Revenue splits, pricing trends, and CAGR estimates
- Supply-side capacity and volume tracking
- Investment analysis and funding benchmarks
2. Qualitative Secondary Research
Objective: Capture strategic direction, innovation signals, and behavioral trends.
Sources include:- Company announcements, roadmaps, and product pipelines
- Publicly available whitepapers, conference abstracts, and academic research
- Regulatory body publications and policy briefs
- Social and media sentiment scanning for early-stage shifts
- Strategic shifts in market positioning
- Unmet needs and white spaces
- Regulatory triggers and compliance impact
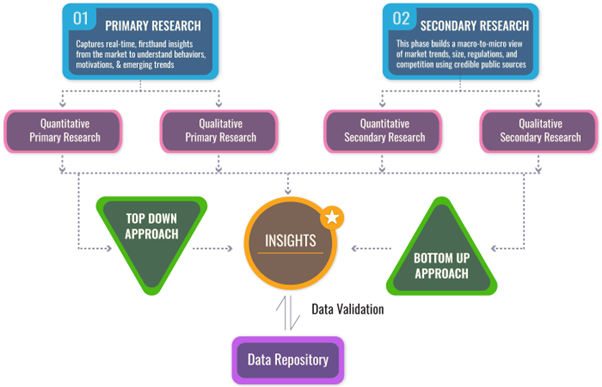
DUAL MODELING: TOP-DOWN + BOTTOM-UP
To ensure robust market estimation, we apply two complementary sizing approaches:
Top-Down Modeling:- Start with broader industry value (e.g., global or regional TAM)
- Apply filters by segment, geography, end-user, or use case
- Adjust with primary insights and validation benchmarks
- Ideal for investor-grade market scans and opportunity mapping
- Aggregate from the ground up using sales volumes, pricing, and unit economics
- Use internal modeling templates aligned with stakeholder data
- Incorporate distributor-level or region-specific inputs
- Most accurate for emerging segments and granular sub-markets
DATA VALIDATION: IN-HOUSE REPOSITORY
We close the loop with proprietary data intelligence built from ongoing projects, industry monitoring, and historical benchmarking. This repository includes:
- Multi-sector market and pricing models
- Key trendlines from past interviews and forecasts
- Benchmarked adoption rates, churn patterns, and ROI indicators
- Industry-specific deviation flags and cross-check logic
- Catches inconsistencies early
- Aligns projections across studies
- Enables consistent, high-trust deliverables

